Some things run in families. When I was a kid, my dad was always walking around with a camera, and even though back then it was analog and you had to print every single picture you took, it did not withhold him from taking thousands of photos. And the apples didn’t fall far from the tree, the love for the art of photography is high in our family.
This is probably why I right away fell in love with microscopy when I first came into contact with it. I only have a vague recollection of most lab classes I took during my undergrad, but this one class I remember vividly. We had to count blood cells under a microscope. I loved looking at the cells and seeing the morphology in such detail. I had no idea this work would become such an essential component of my daily work life, but I sure felt enthusiastic and motivated when the prof leading the course told me that I ‘had a good eye for it.’
In this post, I will highlight some histological imaging techniques for research purposes.
Why look at tissue sections
My work as a cancer immunologist focussed on visualizing what the tumor microenvironment looks like. By studying the tumor cells, surrounding cells, as well as the immune cells that infiltrate the tumor, we aimed to discover what makes an anti-tumor immune response successful. In many areas of biology, looking at what cells you are dealing with is essential to understand the organism you are studying.
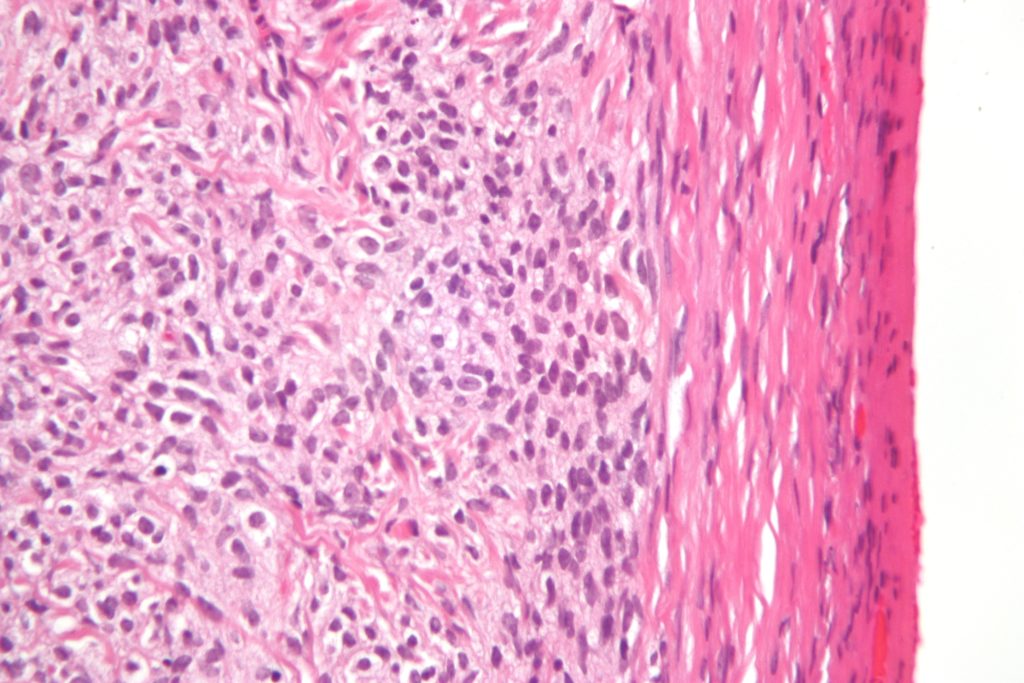
One of the most used staining methods for tissue slides is hematoxylin and eosin (H&E) staining. Hematoxylin is a basic, positively-charged dye that binds basophilic DNA and RNA, coloring the nuclei dark blue/purple. Eosin, on the other hand, is an acidic, negatively-charged dye, which will stain positively-charged amino acids in the cytoplasm pink/orange. The resulting staining pattern allows for excellent visualization of cell morphology and is extensively used by pathologists for medical diagnoses.
Multicolor IHC
When you want to dive deeper into the phenotype of cells, you will have to stain some protein markers on or in the cells. Immunohistochemistry (IHC) is a technique that uses antibodies specific for your protein of interest. You visualize those by adding an enzyme-labeled antibody directed against the species of your primary antibody. The enzyme will convert the chromogen you add to it, leaving a colored spot at the place of that marker. The field has developed fast in the last couple of years, and multicolor IHC increasingly becomes the standard approach.
For multicolor stains, you use various dye/enzyme combinations and/or antibodies derived from different species to demarcate multiple markers at the same time. It works well when you look at specific markers expressed on different types of cells, as you can easily distinguish them. When you want to look at multiple markers on the same cell, it becomes a bit more tricky. Nowadays, there are software packages available allowing you to perform a spectral separation of the colors, but this has its limitations. Also, when you want to look at more than three colors, separation tends to become unreliable.
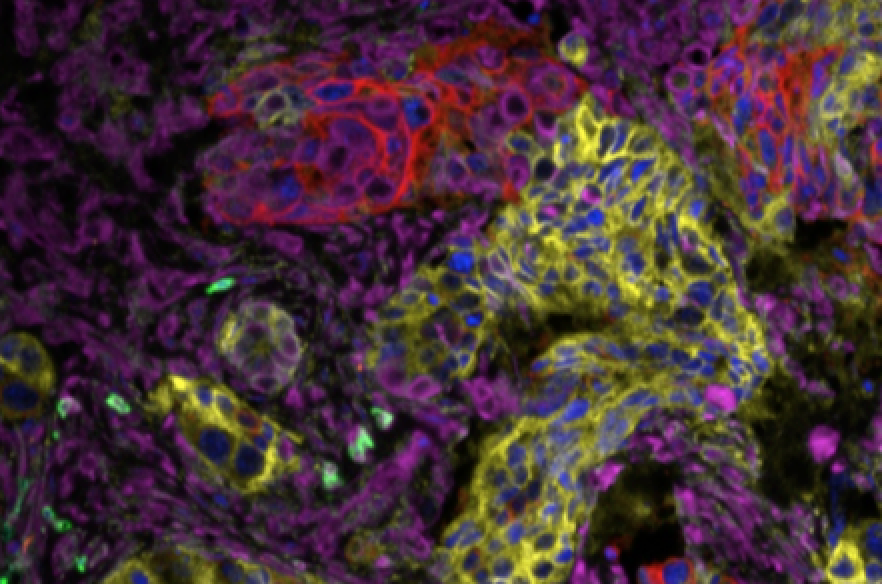
Multicolor IF
For those purposes, immunofluorescence (IF) can be a solution. As each fluorophore emits light at different wavelengths, choosing the fluorophores well allows the visualization of these fluorophores by different detectors, creating a signal in various ‘channels’. You can do this by using antibodies that are directly tagged with a fluorophore, or by using a secondary antibody system.
Signal amplification
Both IHC and IF have a limited detection sensitivity and often require amplification of the signal. For IHC, a commonly used method is to use a polymer of secondary antibodies and enzymes. To each primary antibody, a polymer binds, resulting in more deposit of the staining reagent, increasing the signal. This technique is available for IF as well, but you can get a stronger enhancement of the signal by using tyramide amplification. The principle of this method is very similar to the traditional IHC method. You use a secondary antibody conjugated with an enzyme, and add tyramide-conjugated fluorochromes that deposit on the tissue. After that, you can remove the primary and secondary antibodies, for instance by microwaving the tissue, followed by a restain with the next antibody and fluorochrome combination.
Optimization of IHC and IF
Just like any other work in a research setting, these methods require optimization. Below, I list a couple of important factors to consider when you want to use these techniques. These mainly apply to formalin-fixed paraffin-embedded tissue, but most are applicable to frozen sections as well.
The quality of your work will highly depend on the quality of these parameters:
- Antibodies the specificity of your antibodies is the most essential parameter for successful staining. If the antibody does not bind your target, binds other antigens, or binds only a fraction of your target, you will get unreliable results. For best practice use monoclonal antibodies, as these are less likely to give you significant background. Testing on tissues that you know should be positive and negative are your best controls. Often researchers only perform a ‘leave the primary antibody out’ type of control, but that does not tell you whether the antibody is specific or causes background stain.
- Tissue fixation the duration and chemicals used in the fixation process of the tissue can profoundly affect the results of your staining pattern. Often, optimized protocols for one tissue may not work on differently-processed tissue.
- Age of the tissue time degrades tissue quality, and it is best to use cut slides as soon as possible. Storing slides for extended periods may affect your ability to stain them properly. If you have to store slides longer, the fridge is your best friend.
- Antigen retrieval the fixation process can mask antigens. You will have to revert this process before adding your primary antibody. The most commonly used antigen retrieval methods heat the tissue in an acidic or basic buffer. It will depend on the antigen of choice, the antibody, and your tissue what will be the best method.
As with any technique, you will have to go through some trial and error to perfect the stains. The reward is pretty clear, though, beautiful images that will undoubtedly convince others of the validity of your work.